We started Novocure with the novel insight that electric fields can be harnessed to disrupt cancer cell division selectively and cause cancer cell death. In the 20 years since, we have built an organization of more than 750 colleagues dedicated to delivering Tumor Treating Fields therapy to patients with glioblastoma (GBM) or mesothelioma and dedicated to advancing clinical research and product development programs intended to extend survivals in some of the most aggressive cancers. We made noteworthy advancements in 2019, and we are poised to continue building on this momentum in 2020 and beyond.
We believe we are in a virtuous cycle of execution and innovation supporting the future growth of our company. Tumor Treating Fields therapy is a foundational platform, enabling our efforts to make a difference in cancer. We are focused on growing our commercial business to bring Tumor Treating Fields therapy to as many patients who can benefit as possible. Commercial growth provides us the financial strength and flexibility to increase investments in clinical research and product development programs that will help more patients and propel further growth. We believe we are just beginning our journey.
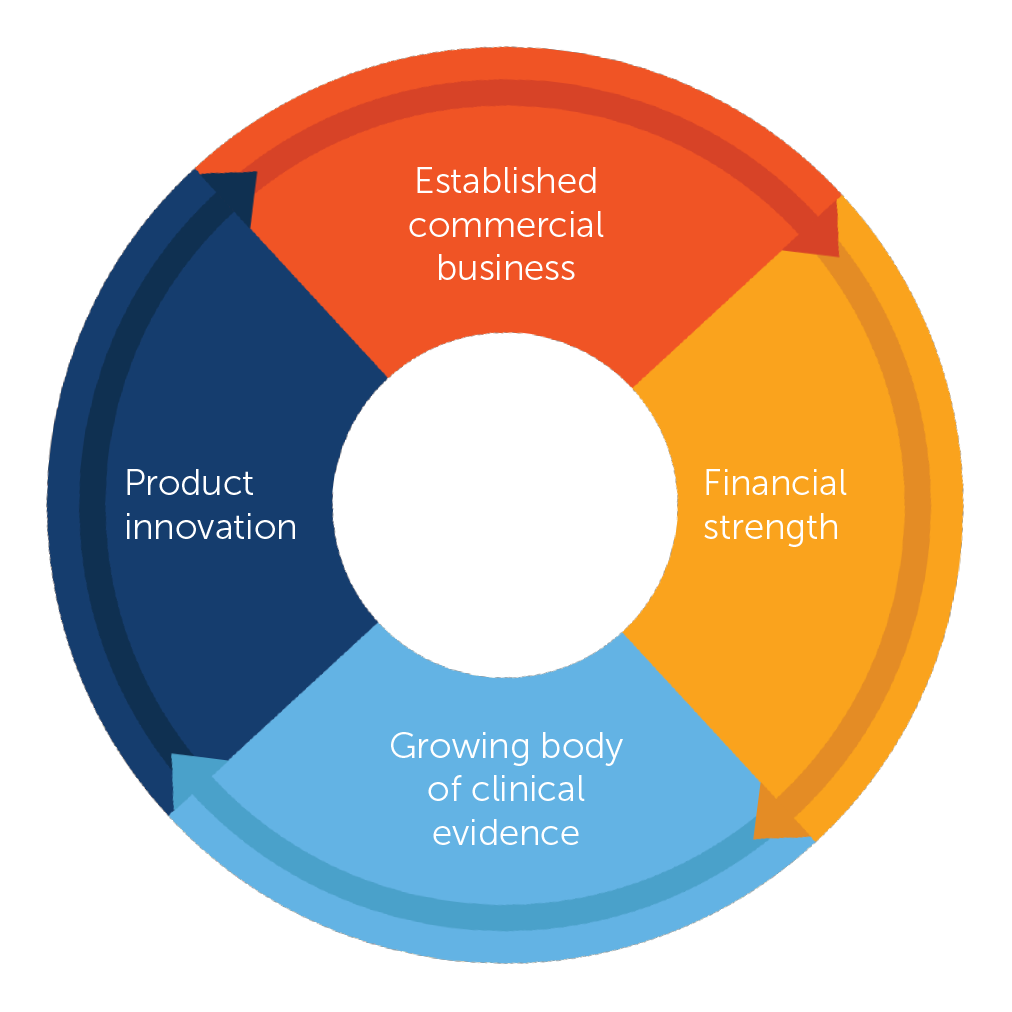
Our 2019 commercial performance reinforces our confidence in the long-term potential of Tumor Treating Fields therapy. In 2019, we received FDA approval via the Humanitarian Device Exemption (HDE) pathway for the treatment of adults with malignant pleural mesothelioma, or MPM, our first FDA-approved torso indication. Medicare established coverage of Optune for the treatment of adults with newly diagnosed GBM. Through our partnership with Zai Lab, we developed our foundation in Greater China. Notably, we accomplished these milestones while generating over $350 million in annual net revenues and adding $80 million in cash to our balance sheet.
With more than 14,000 GBM patients treated to date, we believe Optune has established its central role in the treatment of GBM. As proud as we are of our progress in treating GBM, we believe multiple levers remain to drive near-term growth. We remain focused on working with physicians to expand adoption in our current markets, on laying the groundwork for access in new markets, on extending the duration of therapy for patients and on improving reimbursement for Optune.
Beyond GBM, we launched our second commercial business in 2019 treating patients diagnosed with MPM. Our MPM launch was the first FDA-approved treatment for MPM in over 15 years, and our first approved torso indication. We believe this is an important harbinger of the promise of Tumor Treating Fields in many additional solid tumor indications.
Heading into 2020, our focus is unwavering on growing the awareness and acceptance of Tumor Treating Fields therapy in our approved indications and driving global adoption. With over $320 million of cash on hand at year end, we believe we are well positioned to execute our strategic objectives. We will not be satisfied until all eligible cancer patients are offered an opportunity for long-term quality survival.
Tumor Treating Fields therapy selectively targets the electrical properties of proteins involved in cancer cell division with electric fields tuned to specific frequencies to disrupt mitosis.
Tumor Treating Fields therapy is backed by a growing body of preclinical and clinical evidence. We believe the evidence supports that Tumor Treating Fields’ mechanism of action may be broadly applicable to solid tumor cancers. Our scientific research spans two decades and in all of our preclinical and clinical research to date, Tumor Treating Fields has demonstrated a consistent anti-mitotic effect. Additionally, research has shown that Tumor Treating Fields may have an additive or synergistic effect when combined with certain other cancer therapies without evidence of any dose-limiting, cumulative toxicity. In 2019, there were more than 250 presentations on Tumor Treating Fields at key medical congresses, the majority by independent researchers, underlining the recognition Tumor Treating Fields therapy is gaining in the global oncology community.
We are executing our strategy to make Tumor Treating Fields therapy available in additional indications through phase 2 pilot trials and phase 3 pivotal trials and in current indications through phase 4 post-marketing studies based on a foundation of significant preclinical evidence.
In 2019, we initiated clinical trials studying Tumor Treating Fields in two additional cancers: the INNOVATE-3 trial in recurrent ovarian cancer and the EF-31 trial in gastric cancer. Now, we have four ongoing randomized, phase 3 pivotal trials in brain metastases, non-small cell lung cancer, pancreatic cancer and ovarian cancer, and two phase 2 pilot studies in liver cancer and gastric cancer; creating the potential for substantial market expansion over the next five years.
If approved, the indications in our late-stage pipeline will create a more than 20-fold increase in our addressable U.S. market, alone. We are optimistic about the role Tumor Treating Fields may play in oncology, and we are determined to provide our therapy to cancer patients with a variety of solid tumor types who may benefit from the treatment.
FDA-APPROVED
INDICATIONS
INDICATIONS IN
LATE-STAGE PIPELINE
CASH ON HAND*
AS OF DECEMBER 31, 2019
ISSUED PATENTS AND PENDING APPLICATIONS GLOBALLY
*cash, cash equivalents and short-term investments
Beyond our ongoing preclinical and clinical research, we believe we have a considerable opportunity to improve the efficacy and usability of Optune through product innovation.
A key publication in the Red Journal in April 2019 detailed the dose-efficacy dependence of Tumor Treating Fields. The dose response of Tumor Treating Fields therapy is determined by total energy delivered. Total energy delivered is a function of time on therapy and electric field intensity.
The research published in the Red Journal was pivotal in guiding our product development programs. We are increasing investments in engineering efforts intended both to improve time on therapy and to maximize the energy delivered to patients’ tumors. Specifically, our teams are working to design and develop improvements to our transducer arrays and to our transducer array layout mapping software intended to increase Tumor Treating Fields intensity and, as a result, survival.
We believe innovation has the potential to improve patient outcomes and to extend our intellectual property protection into the future as we invent enhancements to our products. Our commitment to innovation resulted in 33 new patent applications in 2019, alone. Supported by our financial strength, we remain committed to investing in clinical research and product development to extend survival in our current and future indications and to unlock future value for our patients, employees and shareholders.
Re-imagining the possible is the essential theme of Novocure’s story.
We have a remarkable 20-year history of building Novocure from Professor Palti’s vision into an established global oncology company. As proud as we are of the accomplishments of our first 20 years, the responsibility to treat more patients diagnosed with some of the most aggressive cancers remains before us. Our mission is grounded in this reality.
Each day, we see our patients for the people they are with families, friends, hopes and dreams. Each day, we see the physical and emotional pain cancer causes. Each day, we do not look away, rather we look forward to work to provide access to Tumor Treating Fields therapy to more patients in our approved indications, to complete clinical research in new indications, and to further improve the efficacy of Tumor Treating Fields therapy through product development.
Thank you for your continued support.
Bill Doyle,
Executive Chairman

Asaf Danziger,
CEO