Since our IPO in 2015, we delivered double-digit or greater revenue growth every year, increased our gross margins from the mid-40s to the mid-70s and invested over $250 million in research and development. In 2019, we began to generate cash flow from operations and reported our first two quarters of profitability. We believe our financial strength positions us well to achieve our strategic objectives, and we remain committed to maintaining a balanced focus across growth, profitability and liquidity.

GROWTH IN
ACTIVE PATIENTS
GROWTH IN RESEARCH &
DEVELOPMENT INVESTMENTS
GROWTH IN
NET REVENUES
CASH ON HAND* ADDED
TO THE BALANCE SHEET
*cash, cash equivalents and short - term investments
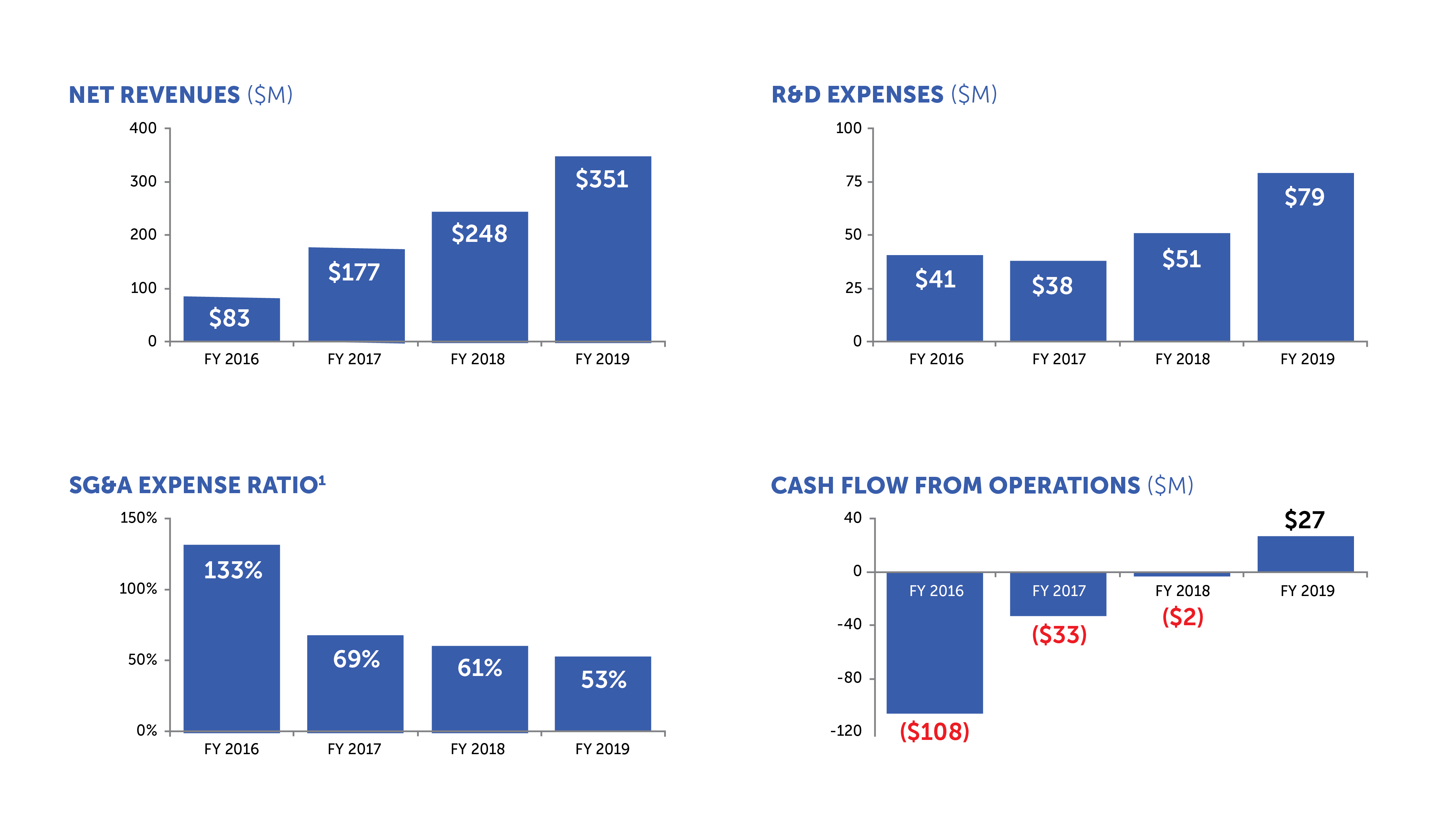
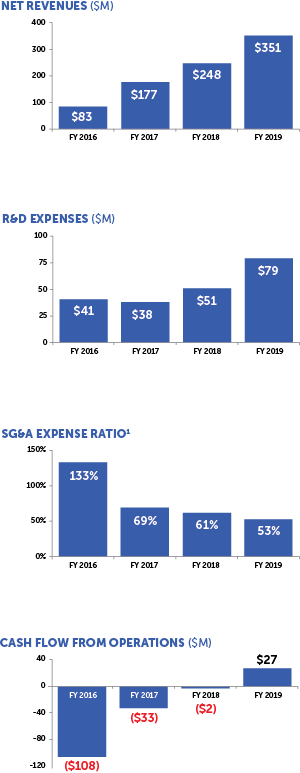
¹ SG&A Expense Ratio equals total selling, general and administrative expenses divided by total net revenues in the period
| Year ended December 31, | U.S. dollars in thousands | 2019 | 2018 | 2017 | 2016 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Net revenues | $ 351,318 | $ 248,069 | $ 177,026 | $ 82,888 | |||||||
| Cost of revenues | 88,606 | 80,048 | 55,609 | 39,870 | |||||||
| Impairment of field equipment | – | – | – | 6,412 | |||||||
| Gross profit | 262,712 | 168,021 | 121,417 | 36,606 | |||||||
| Research, development and clinical trials | 79,003 | 50,574 | 38,103 | 41,467 | |||||||
| Sales and marketing | 96,675 | 77,663 | 63,528 | 59,449 | |||||||
| General and administrative | 87,948 | 73,456 | 59,114 | 51,007 | |||||||
| Total operating expenses | 263,626 | 201,693 | 160,745 | 151,923 | |||||||
| Operating income (loss) | (914) | (33,672) | (39,328) | (115,317) | |||||||
| Financial expenses (income), net | 7,910 | 12,270 | 9,169 | 6,147 | |||||||
| Income (loss) before income taxes | (8,824) | (45,942) | (48,497) | (121,464) | |||||||
| Income taxes | (1,594) | 17,617 | 13,165 | 10,381 | |||||||
| Net income (loss) | $ (7,230) | $ (63,559) | $ (61,662) | $ (131,845) | |||||||
| Basic and diluted net income (loss) per ordinary share | $ (0.07) | $ (0.69) | $ ((0.70) | $ (1.54) |

—Todd Longsworth,
General Counsel
William F. Doyle
Executive Chairman
Yoram Palti, M.D., Ph.D.
Founder
Ely Benaim, M.D.
Chief Medical Officer
Mike Ambrogi
Chief Operating Officer
Pritesh Shah
Chief Commercial Officer
Asaf Danziger
Chief Executive Officer
Todd Longsworth
General Counsel
Uri Weinberg, M.D., Ph.D.
Chief Science Officer
Wilco Groenhuysen
Chief Financial Officer
William F. Doyle
Executive Chairman
David T. Hung
Jeryl Hilleman
Kinyip Gabriel Leung
Asaf Danziger
Chief Executive Officer
Martin J. Madden
Sherilyn D. McCoy
William A. Vernon
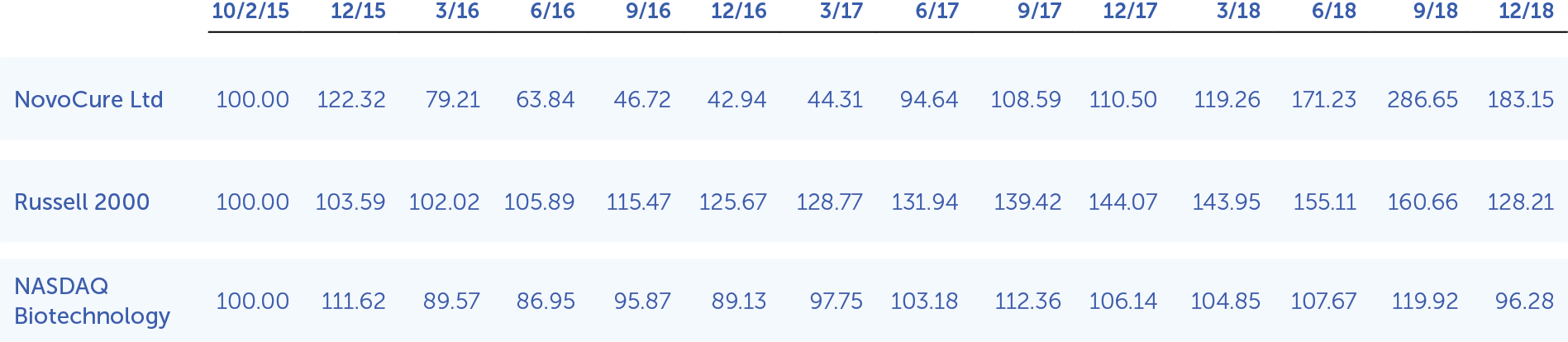
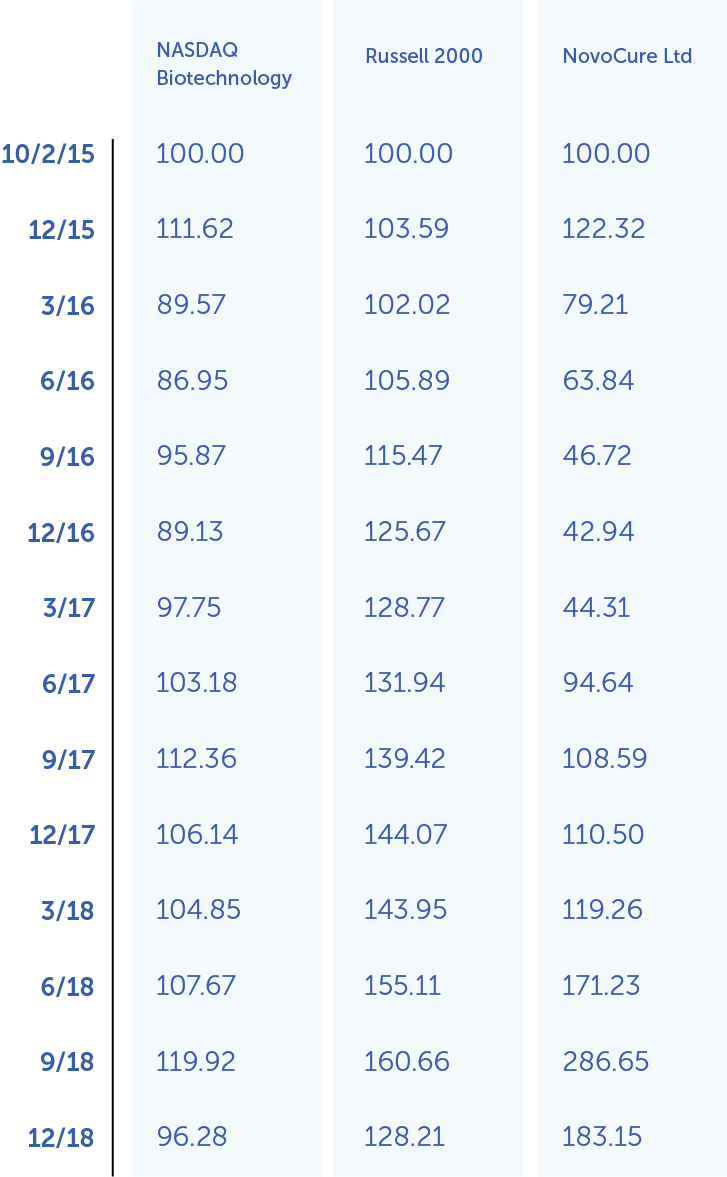
The following performance graph is being furnished as part of this annual report and shall not be deemed "filed" with the SEC or incorporated by reference into any of our filings under the Securities Act of 1933, as amended (the "Securities Act"), or the Exchange Act, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
The following graph shows the total shareholder return of an investment of $100 in cash at market close on October 2, 2015 (the first day of trading of our ordinary shares) through December 31, 2019 for (1) our ordinary shares, (2) the Russell 2000 Index, and (3) the Nasdaq Biotechnology Index. Pursuant to applicable SEC rules, all values assume reinvestment of the full amount of all dividends; however, no dividends have been declared on our ordinary shares to date. The shareholder return shown on the graph below is not necessarily indicative of future performance, and we do not make or endorse any predictions as to future stockholder returns.
Among NovoCure Ltd, the Russell 2000 Index, and the Nasdaq Biotechnology Index
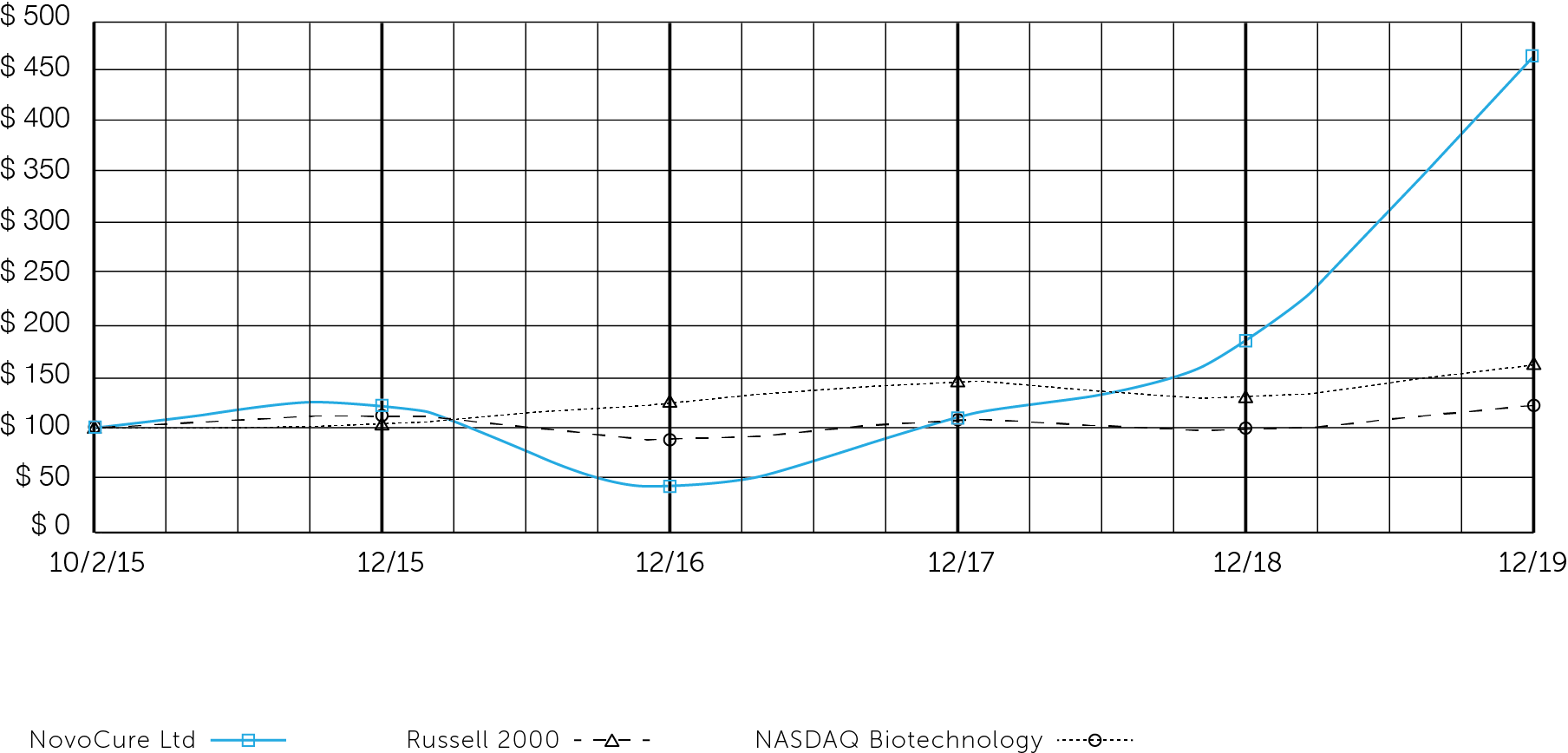
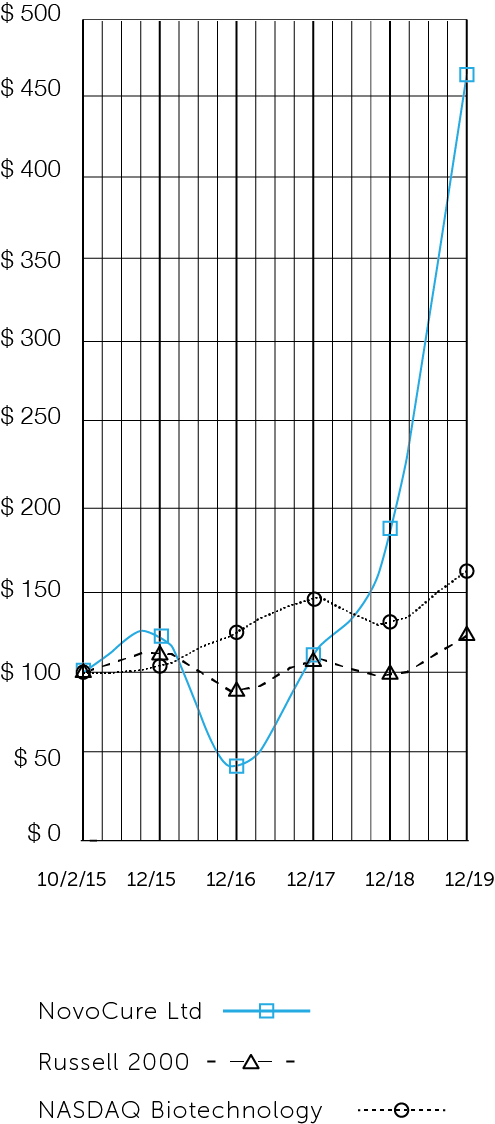
*$100 invested on 10/2/15 in stock or 9/30/15 in index, including reinvestment of dividends. Fiscal year ending December 31.
Copyright© 2019 Russell Investment Group. All rights reserved.
INDICATIONS
Optune is intended as a treatment for adult patients (22 years of age or older) with histologically-confirmed glioblastoma multiforme (GBM).
Optune with temozolomide is indicated for the treatment of adult patients with newly diagnosed, supratentorial glioblastoma following maximal debulking surgery, and completion of radiation therapy together with concomitant standard of care chemotherapy.
For the treatment of recurrent GBM, Optune is indicated following histologically-or radiologically-confirmed recurrence in the supratentorial region of the brain after receiving chemotherapy. The device is intended to be used as a monotherapy, and is intended as an alternative to standard medical therapy for GBM after surgical and radiation options have been exhausted.
Optune Lua is indicated for the treatment of adult patients with unresectable, locally advanced or metastatic, malignant pleural mesothelioma (MPM) to be used concurrently with pemetrexed and platinum-based chemotherapy.
CONTRAINDICATIONS
Do not use Optune in patients with GBM with an implanted medical device, a skull defect (such as, missing bone with no replacement), or bullet fragments. Use of Optune together with skull defects or bullet fragments has not been tested and may possibly lead to tissue damage or render Optune ineffective. Do not use Optune Lua in patients with MPM with implantable electronic medical devices, such as pacemakers or implantable automatic defibrillators, etc.
Use of Optune for GBM or Optune Lua for MPM together with implanted electronic devices has not been tested and may lead to malfunctioning of the implanted device.
Do not use Optune for GBM or the Optune Lua for MPM in patients known to be sensitive to conductive hydrogels. Skin contact with the gel used with Optune or Optune Lua may commonly cause increased redness and itching, and may rarely lead to severe allergic reactions such as shock and respiratory failure.
WARNINGS AND PRECAUTIONS
Optune and Optune Lua can only be prescribed by a healthcare provider that has completed the required certification training provided by Novocure®.
The most common (≥10%) adverse events involving Optune in combination with chemotherapy in patients with GBM were thrombocytopenia, nausea, constipation, vomiting, fatigue, convulsions, and depression.
The most common (≥10%) adverse events related to Optune treatment alone in patients with GBM were medical device site reaction and headache. Other less common adverse reactions were malaise, muscle twitching, and falls related to carrying the device.
The most common (≥10%) adverse events involving Optune Lua in combination with chemotherapy in patients with MPM were anemia, constipation, nausea, asthenia, chest pain, fatigue, device skin reaction, pruritus, and cough.
Other potential adverse effects associated with the use of Optune Lua include: treatment related skin toxicity, allergic reaction to the plaster or to the gel, electrode overheating leading to pain and/or local skin burns, infections at sites of electrode contact with the skin, local warmth and tingling sensation beneath the electrodes, muscle twitching, medical site reaction and skin breakdown/skin ulcer.
If the patient has an underlying serious skin condition on the treated area, evaluate whether this may prevent or temporarily interfere with Optune or Optune Lua treatment. Do not prescribe Optune or Optune Lua for patients that are pregnant, you think might be pregnant or are trying to get pregnant, as the safety and effectiveness of Optune and Optune Lua in these populations have not been established.
Please go to Optune.com to see the Optune Instructions For Use (IFU) for complete information regarding the device's indications, contraindications, warnings, and precautions.
Please go to OptuneLua.com to see the Optune Lua IFU for complete information regarding the device's indications, contraindications, warnings, and precautions.
In addition to historical facts or statements of current condition, this annual report may contain forward-looking statements. Forward-looking statements provide Novocure’s current expectations or forecasts of future events. These may include statements regarding anticipated scientific progress on its research programs, clinical trial progress, development of potential products, interpretation of clinical results, prospects for regulatory approval, manufacturing development and capabilities, market prospects for its products, coverage, collections from third-party payers and other statements regarding matters that are not historical facts. You may identify some of these forward-looking statements by the use of words in the statements such as “anticipate,” “estimate,” “expect,” “project,” “intend,” “plan,” “believe” or other words and terms of similar meaning. Novocure’s performance and financial results could differ materially from those reflected in these forward-looking statements due to general financial, economic, regulatory and political conditions as well as issues arising from the COVID-19 pandemic and other more specific risks and uncertainties facing Novocure such as those set forth in its Annual Report on Form 10-K filed on February 27, 2020 and its Quarterly Report on Form 10-Q filed on April 30, 2020 with the U.S. Securities and Exchange Commission. Given these risks and uncertainties, any or all of these forward-looking statements may prove to be incorrect. Therefore, you should not rely on any such factors or forward-looking statements. Furthermore, Novocure does not intend to update publicly any forward-looking statement, except as required by law. Any forward-looking statements herein speak only as of the date hereof. The Private Securities Litigation Reform Act of 1995 permits this discussion.